WINTHER Summary
The WINTHER trial*, NCT01856296, led by investigators from Vall d’Hebron Institute of Oncology - VHIO (Spain), Chaim Sheba Medical Center (Israel) (Raanan Berger), Gustave Roussy (France) (Jean-Charles Soria), Centre Léon Bérard (France) (Pierre Saintigny), Segal Cancer Centre, McGill University (Canada) (Wilson H. Miller), UT MD Anderson Cancer Center (USA) (Jordi Rodon and Apostolia-Maria Tsimberidou) and University of California San Diego, Moores Cancer Center (USA) (Razelle Kurzrock), aimed to expand precision oncology to patients with advanced solid tumors that progressed after treatment with standard therapies.
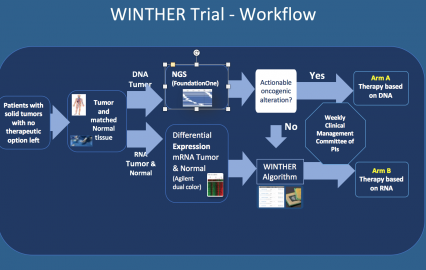
For the first time in the clinic, the WINTHER trial applied transcriptomics (RNA expression testing) to tailor precision medicine in oncology to a greater number of patients based on the increased expression of RNA in tumors compared to normal tissues.
303 patients were enrolled in WINTHER; 107 of whom were ultimately treated according to recommendations made by a committee of cancer experts spanning five countries. These patients had been heavily pretreated, with one quarter having received five or more prior lines of therapy. Of the 107 patients treated, 69 received treatment based on DNA mutation profiling, and 38 based on RNA profiling. Overall, the WINTHER trial succeeded in matching personalized therapy to 35% of patients with advanced cancer.
“The strategy employed in WINTHER resulted in a higher proportion of patients treated than in many precision medicine trials. Previous studies have identified potential treatments for between 5% and 25 % of patients based on DNA profiling alone, our findings represent an important step toward delivering on the true promise of precision medicine in oncology,” said Richard L. Schilsky, Chairman WIN Consortium and Chief Medical Officer of ASCO.
In this trial, patients were first evaluated for targetable alterations in cancer driver genes. Those who were not matched to drugs based on DNA alterations received a treatment tailored to the differences in gene expression between patients’ tumors and normal tissues which were assessed using a patented algorithm developed by the WIN Consortium. Comparisons with normal tissues proved essential due to highly variable RNA expression between patients and across normal tissue types. The WINTHER researchers showed that RNA expression can be used to expand personalized therapy options for patients and that normal tissue biopsy is safe and accepted by patients.
Patients who received therapy optimally tailored to their respective DNA alterations, or consistent with the algorithm recommendation for RNA guided treatment, responded better. Patients with a good performance status and a high degree of matching had a significantly longer median overall survival of 25.8 months versus 4.5 months for others.
There was also a correlation between degree of matching and progression-free survival independent of the number of prior therapies.
WINTHER Acknowledgement
WINTHER received funding from the European Union Seventh Framework Program (FP7/2007-2013 under grant agreement n°306125), ARC Foundation for cancer research (France), Pfizer Oncology, Lilly France SAS, and Novartis Pharmaceuticals Corporation.
Dr. Razelle Kurzrock speaks about the results of the WINTHER trial at WIN 2019
June 23, 2019
https://youtu.be/zqmnMrY3G14
WINTHER presentations
Trial results were presented by Dr. Razelle Kurzrock at WIN 2019 Symposium on June 23-24, 2019 in Paris.
Trial in progress update was presented at ASCO 2018 by Dr.Jordi Rodon from Vall d’Hebron Institute of Oncology, Barcelona, Spain.
Trial in progress update was at WIN 2018 Symposium by Dr. Razelle Kurzrock from University California San Diego Moores Cancer Center, USA.
WINTHER Results in Nature Medicine
Published in Nature Medicine, results of WINTHER, the first study pioneered by the WIN Consortium - - Genomic and transcriptomic profiling expands precision cancer medicine: the WINTHER trial - shows that RNA profiling matches more patients with advanced cancer to personalized therapies than standard testing for DNA mutations in tumors.
Download pdf
Further biological data available upon request at:
trialadministrator@winconsortium.org
WINTHER in Annals of Oncology
«Challenges in initiating and conducting personalized cancer therapy trials: perspectives from WINTHER, a Worldwide Innovative Network (WIN) Consortium trial»
June 1, 2015
Download pdf
WIN Consortium Applies Transcriptomics to Bolster Patient Matching in Precision Oncology Study
«WIN Consortium Applies Transcriptomics to Bolster Patient Matching in Precision Oncology Study»
June 5, 2018
CHICAGO (GenomeWeb) – The combination of DNA and RNA analysis allowed more cancer patients to be matched to precision medicine options than would have been possible based on DNA analysis only, a study presented at the American Society of Clinical Oncology’s annual meeting showed.
Although the WINTHER study, conducted by the WIN Consortium, did not meet a prespecified clinical benefit endpoint, a blinded, post-hoc analysis showed that when patients received treatments they were most likely to benefit from, as determined by high matching scores, they lived significantly longer compared to those who did not get the top-matched therapies. The WINTHER investigators said the data demonstrate the importance of integrating transcriptomics into precision oncology trials alongside DNA analysis.
Download pdf
WINTHER first large-scale basket prospective trial incorporating transcriptomics for treatment decision provides unique data
'Synthetic lethality-mediated precision oncology via the tumor transcriptome' Cell Press, April 14, 2021
Precision oncology has made significant advances, mainly by targeting actionable mutations in cancer driver genes. Aiming to expand treatment opportunities, recent studies have begun to explore the utility of tumor transcriptome to guide patient treatment. Here, we introduce SELECT (synthetic lethality and rescue-mediated precision oncology via the transcriptome), a precision oncology framework harnessing genetic interactions to predict patient response to cancer therapy from the tumor transcriptome. SELECT is tested on a broad collection of 35 published targeted and immunotherapy clinical trials from 10 different cancer types.
It is predictive of patients’ response in 80% of these clinical trials and in the recent multi-arm WINTHER trial.
The predictive signatures and the code are made publicly available for academic use, laying a basis for future prospective clinical studies.
Download pdf
WINTHER Study
WINTHER Leaders

Razelle Kurzrock
Chief Medical Officer, WIN Consortium Prof. of Medicine, Director, Center for Precision Oncology MCW Cancer Center and Linda T. & John A. Mellowes Chair of Precision Oncology (USA)

Jordi Rodon
Associate Professor, Dept. of Investigational Cancer Therapeutics, The University of Texas MD Anderson Cancer Center (USA)


Raanan Berger
Director, Institute of Oncology, Sheba Medical Center (Israel)

Wilson Miller
Director, Laboratory Research, Clinical Research Unit, Developmental Therapeutics Program, Professor, Division of Oncology, Segal Cancer Center, Jewish General Hospital (Canada)

Aliza Ackerstein
Phase 1, Clinical Research Manager, Sheba Medical Center (Israel)

Pierre Saintigny
Medical Oncologist, Professor of Medicine at University Claude Bernard Lyon, Coordinator of the Translational Medicine Unit at Centre Léon Bérard (France)


Apostolia-Maria Tsimberidou
Vice Chair, WIN Consortium; Professor, Department of Investigational Cancer Therapeutics, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center (USA)

